We welcome you to WorldLab 2024!

After a first participation in 2022 we are back at WorldLab!
Meet the team and our product portfolio for all your calprotectin needs, join our Workshop.
From patient to the lab, BÜHLMANN Laboratories supports you each step of the way.
BÜHLMANN Highlights
Calprotectin portfolio
BÜHLMANN has launched its first assay for fecal calprotectin testing almost 20 years ago and is considered today as the gold-standard for IBD diagnosis and monitoring.
Our range of assays covers all customer needs, from the single patient’s home to the high-throughput laboratories.
Grow your lab with our portfolio! BÜHLMANN calprotectin assays guarantee the same standardization across all platforms.
Our calprotectin product portfolio is the most extensive in the industry, featuring solutions for every requirement. We offer products ranging from the automated, random-access FDA-cleared BÜHLMANN fCAL® turbo and fCAL® ELISA, recognized as a market leader, to the Quantum Blue® rapid test and the IBDoc® calprotectin home test. The BÜHLMANN calprotectin assays feature in more than 100 clinical publications thereby demonstrating its reliability and the diagnostic quality.
BÜHLMANN also provides the most innovative stool preparation device, underscoring the critical role of pre-analytics in calprotectin measurement. The CALEX® Cap, CALEX® Cap Collection Set and CALEX® Valve offer very easy to use and hygienic stool collection and all are pre-filled with the BÜHLMANN extraction buffer.
CALEX® Cap – a new generation
The CALEX® Cap stool preparation device offers an easy to use and hygienic stool sampling with the BÜHLMANN extraction buffer. CALEX® Cap is fully compatible with total laboratory automation (TLA) solutions for maximum workflow optimization. Experience the simplicity of stool sampling and its seamless integration in your laboratory testing workflow, with the new extraction tutorial for professionals.
Visit our Educational Workshop:
Fecal Calprotectin: A Biomarker for Gastrointestinal Inflammation in IBD – Clinical Insights and Efficient Laboratory Workflow
Learn about calprotectin, all its possible aspects, from research to its clinical value and the assay set up in the lab.
Date: 28th May 2024
Time: 14.30 to 15.30
Number: EduW 12
ROOM B
PROGRAMME:
14.30 – Calprotectin: Insights into biological function & biochemical analysis – Alexander Ohmann
14.50 – Clinical value of calprotectin in the diagnosis and management of patients suffering from IBD – Prof. Jordi Guardiola
15.10 – Fecal calprotectin testing in routine lab work: the experience from a reference center – Rouba Trad
15.25 – Q&A session
Our Speakers:

Prof. Jordi Guardiola (Belvitge Hospital, Barcelona, Spain)
Prof. Jordi Guardiola is Head of the Digestive Diseases Department of the Hospital Universitari de Bellvitge (Barcelona), Director of the IBD Unit of that hospital and Associate Professor of medicine at the University of Barcelona.
He is the past President of the “Catalan Society of Gastroenterology”. Dr. Guardiola is an expert advisor for the “Spanish Working Group for Crohn’s Disease and Ulcerative Colitis” (GETECCU) and had been member of the Steering Committee of this organization. His Scientific work contributed to more than 150 peer-reviewed scientific papers in international journals, more than 450 communications to congresses and multiple lectures. He has been PI in more than 40 clinical trials.

Mrs Rouba Trad (Clemenceau Medical Center, Beirut, Lebanon)
Rouba Trad has been the Pathology and Lab Manager at Clemenceau Medical Center -Lebanon since 2009, where she served as well a laboratory consultant to support affiliates across the region. After commissioning the laboratory into automation, she worked on operational excellence to ensure sustainability and financial viability within any faced challenges while maintaining quality standards. She is an invited speaker to many scientific congresses, events regarding Lab automation, optimization and total value of ownership. Rouba holds a BS in Medical Laboratory Sciences and a Master’s in Public Health and Policy with a concentration in Lab Management from American University of Beirut-Lebanon.

Alexander Ohmann, PhD (BÜHLMANN Laboratories AG, Switzerland)
Dr. Alexander Ohmann holds a diploma degree in Physics from the TU Dresden, Germany, and a PhD in Biophysics from the University of Cambridge, UK. In his academic career he applied DNA nanotechnology to design synthetic, DNA-built enzymes and proteins as a novel class of patient-specific therapeutics.
In 2021, he joined the BÜHLMANN Development department focusing on the development of turbidimetric immunoassays to assess the inflammatory status of patients. As a nanotechnology and clinical chemistry specialist, he has since advanced to a Senior Scientist position and has become a key driver in further expanding BÜHLMANN’s calprotectin expertise.

Chair:
Christian Gerhold, PhD (BÜHLMANN Laboratories AG, Switzerland)
Dr. Christian-Benedikt Gerhold studied Biochemistry as well as macromolecular science in Bayreuth, Germany and pursued his PhD in structural biology and epigenetics at the Gene Center of the LMU in Munich. During his Post-Doctoral research, he was working on genome integrity and nuclear actin at the Friedrich-Miescher Institute in Basel.
He joined BÜHLMANN 2017 as a Senior Scientist in the Corporate Research group and was appointed Head of Corporate Research in 2020. Since 2021 Dr. Gerhold leads the development department of BÜHLMANN Laboratories as CTO and is thus responsible for Product Development and Validation.
BÜHLMANN Posters
Our team will be presenting these posters this year at WorldLab 2024!
P1550 A RECOMBINANT FUSION PROTEIN THAT MIMICS THE INFLAMMATORY BIOMARKER CALPROTECTIN AS A TOOL TO HARMONIZE MRP-8/MRP-14 IMMUNOASSAYS

BÜHLMANN Calprotectin Assays – From niche to full automation
BÜHLMANN is the gold standard for IBD diagnosis and monitoring offering the largest product portfolio of calprotectin testing and TDM rapid tests for biologics in IBD.
The product portfolio includes the FDA cleared BÜHLMANN fCAL® turbo for high throughput testing. The immuno-turbidimetric fecal calprotectin assay BÜHLMANN fCAL® turbo provides flexible and random-access applications on all major clinical chemistry platforms. The assay allows short turnaround time from fecal sample to the reportable result.
The combination of the test with the CALEX® Cap device or the fecal collection kit CALEX® Cap Collection Set strongly simplifies the cumbersome procedure for stool pre-analytics and is adapted to be applied on clinical chemistry analyzers. BÜHLMANN also offers the FDA cleared market leader Fecal calprotectin ELISA (BÜHLMANN fCAL® ELISA) and the Quantum Blue® calprotectin rapid tests. Moreover, the portfolio includes IBDoc®, a highly successful inflammatory bowel disease home test for patients using an app that transforms smartphones into a calprotectin test reading device.

The new assay BÜHLMANN fPELA® turbo and the well-established BÜHLMANN fCAL® turbo together with the CALEX® Cap have revolutionized fecal testing. Both turbidimetric immunoassays can be easily applied for a wide choice of clinical chemistry analysers, providing TLA with fast turn-around-time (TAT) and improve laboratory workflow. The combined use of these products makes reliable results possible for an extended measuring range.
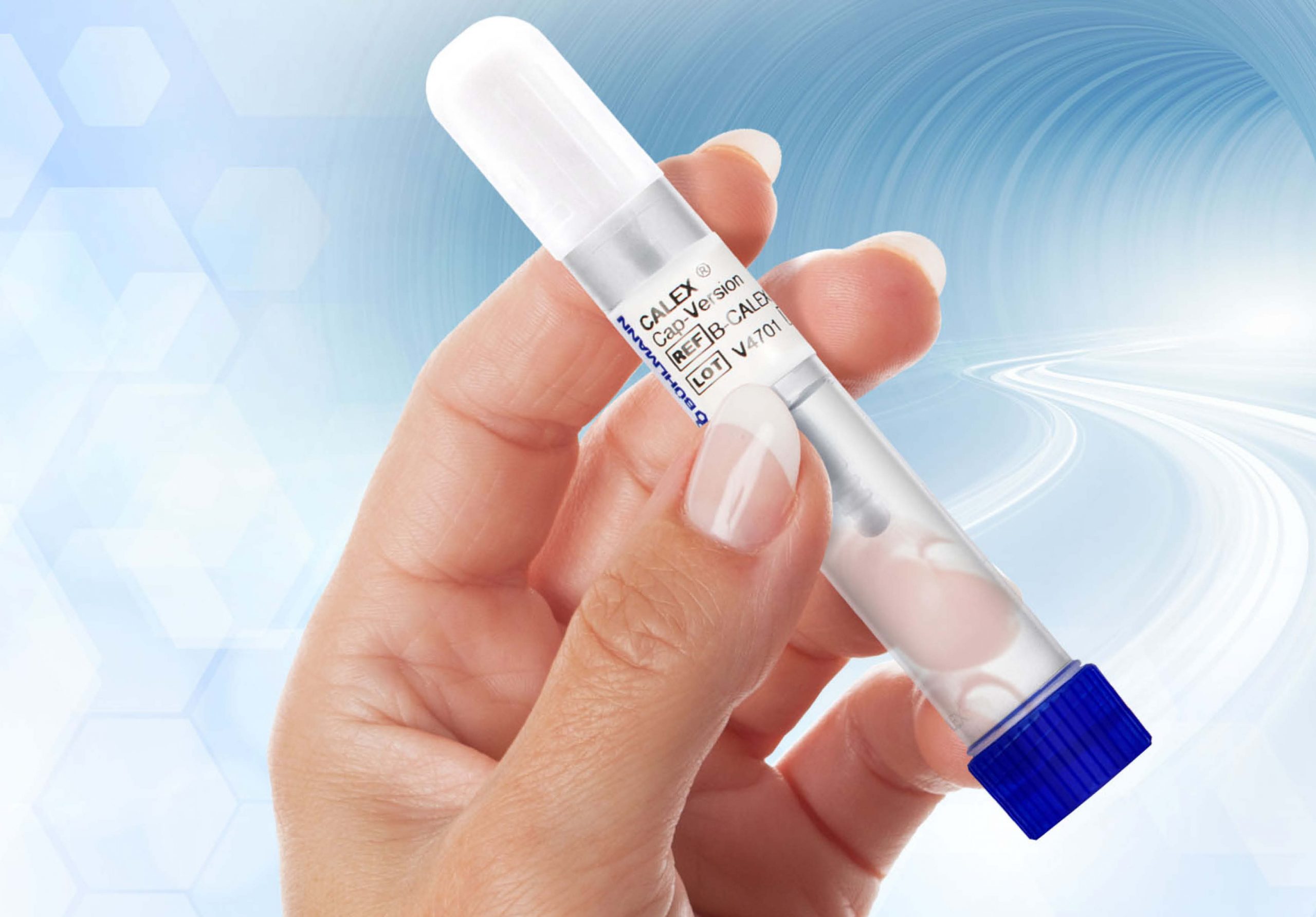
The BÜHLMANN CALEX® Cap device simple design and buffer offer the user high safety as well as high stability of stool samples. With the device, stool collection and extraction in the laboratory environment is efficient, convenient, and hygienic. Furthermore, the CALEX® Cap is designed to be fully compatible with total laboratory automation (TLA) solutions.

The CALEX® Cap Collection Set further reduces the laboratory task of stool preparation procedure for fecal calprotectin and pancreatic elastase testing with BÜHLMANN assays. The CALEX® Cap Collection Set offers efficient, convenient, and hygienic direct stool collection.

The BÜHLMANN sCAL® turbo assay is a flexible and powerful tool to be applied on most clinical chemistry analyzers for measurement of serum calprotectin. The turbidimetric technology allows rapid and flexible random-access options offering high throughput in the routine laboratory. Another major asset of the BÜHLMANN sCAL® turbo assay is the dramatically reduced hands-on time compared to other technologies, allowing serum calprotectin result reporting within a very short time.

The BÜHLMANN Quantum Blue® rapid test combines the ease and speed of lateral flow technology, with full quantification by means of a small reading device. The Quantum Blue® Reader provides a quantitative value within minutes. The BÜHLMANN product line offers two different fecal calprotectin test ranges suitable for inflammatory bowel disease diagnosis and therapy follow-up of Crohn or Ulcerative Colitis patients. Besides calprotectin assays, BÜHLMANN offers the possibility for therapeutic drug monitoring for biologics in IBD on the Quantum Blue® Reader. The Quantum Blue® interface offers the connection to Laboratory and Hospital Information Systems (LIS/HIS).

IBDoc® is the first in vitro diagnostic home testing device measuring the inflammatory marker fecal calprotectin at home. The IBDoc® mobile app turns your smartphone into a test cassette reader using state of the art image processing. Stool preparation is performed using the CALEX® Valve that is characterized by its simple and convenient handling of stool samples. The secure data connection between the IBDoc® mobile app and the IBDoc® Portal allows Health Care Professionals to directly monitor Crohn’s or Ulcerative Colitis’ patient results. The IBDoc® Portal also includes an application programming interface (API) that directly transfers test results into electronic patient records (EHR) or laboratory information systems (LIMS) via a common web standard or HL7 based interface.

BÜHLMANN launched its first assay for fecal calprotectin testing almost 20 years ago and is today considered as the gold standard with the BÜHLMANN fCAL® ELISA, a trusted tool applied in numerous large clinical trials. Two protocols are available with the BÜHLMANN fCAL® ELISA to cover different calprotectin ranges for Crohn and Ulcerative Colitis diagnosis and monitoring.