Fecal Calprotectin Assays
Multiple Applications, Evolving Assays for Inflammation to Meet Your Needs
Trust Your Calprotectin Results – Robust, Standardized, Validated and Published
Which Assay is Best for You?
The BÜHLMANN Calprotectin Assay product line has a range of solutions that services all types of users, from the subject’s home test to the high throughput labs running thousands of samples. We have a variety of test formats to best suit your needs and no matter which assay you choose results are reliable, accurate, and consistent across all the board.
Fast, Flexible, Random Access
Automation solution for mid to high throughput laboratories which can be applied on most common routine chemistry analyzers.
Gold Standard
For higher throughput, batch testing laboratory use (automation available on the multiple ELISA Processing Systems).
Rapid and Quantitative
Lateral Flow Assay for Calprotectin and Therapeutic Drug Monitoring for low throughput use.

BUHLMANN fCAL® turbo
Fast, Flexible, Random Access
BÜHLMANN fCAL® turbo is a flexible turbidimetric assay for fecal calprotectin applicable on most major clinical chemistry platforms.
The technology of immunoturbidimetry implements a further milestone in fecal calprotectin quantification. It allows for automated, rapid and flexible random access use as well as being the ideal solution for high throughput applications in the routine laboratory.
The BÜHLMANN fCAL® turbo is a turbidimetric immunoassay based on the standardization of the BÜHLMANN fCAL® ELISA, which is globally established in laboratories as the gold standard for calprotectin measurements.
Speed: results within 10 minutes
Quality: This assay is standardized against the well established BÜHLMANN fCAL® ELISA and the PETIA methodology enhances precision and reproducibility.
Flexibility: Immunoturbiometric assays can be applied to most clinical chemistry platforms, allows random access workflow which can be directly integrated into the existing laboratory infrastructure.
Range: 20 – 8000 μg/g
Based on the established BÜHLMANN standards.
Use with CALEX® Cap device: Combined with the CALEX® CAP extraction device further simplifies the fecal calprotectin diagnostic workflow thus reducing hands-on time dramatically.
Validated Applications
| Abbott | Architect c-series, Alinity |
| Beckman | AU Series (AU400/640), AU480, AU680, AU5800, DxC 700AU, DxC 600/800 |
| Mindray | BS-200E, BS-240/240Pro, BS-380, BS-400 |
| Ortho | Vitros 5600 |
| Roche | cobas c501/502, c503, c701/702, c111 |
| Siemens | Advia 1650/1800, 2400, XPT, Atellica CH 930 |
| ThermoFisher | Konelab30i, Indiko/Indiko Plus |
| The Binding Site | Optilite |
| Horiba ABX | Pentra 400 |
| Biosystems | BA200 |
*validation ongoing; **validation planned
Is your analyzer not listed? Please contact support@buhlmannlabs.ch and we will find a solution for your analyzer and your needs.
Method Comparison
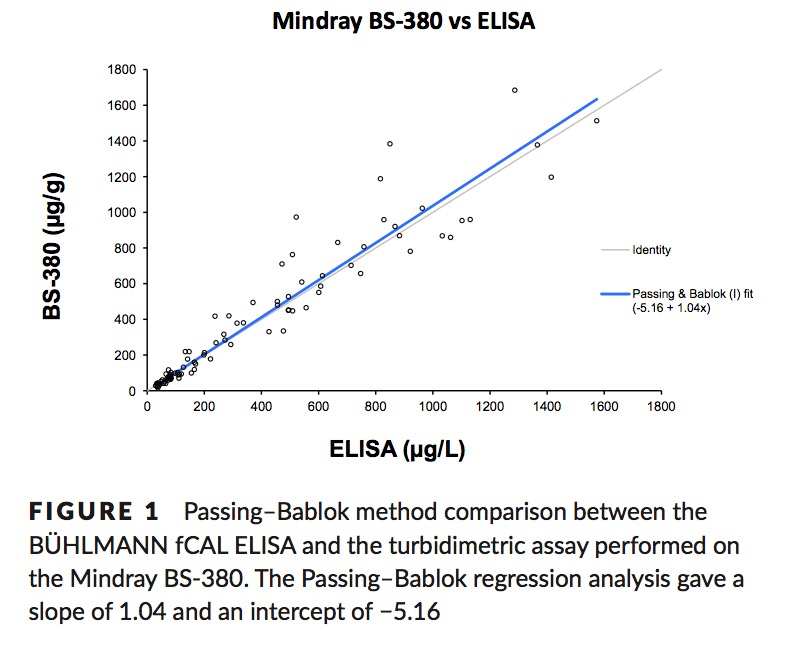
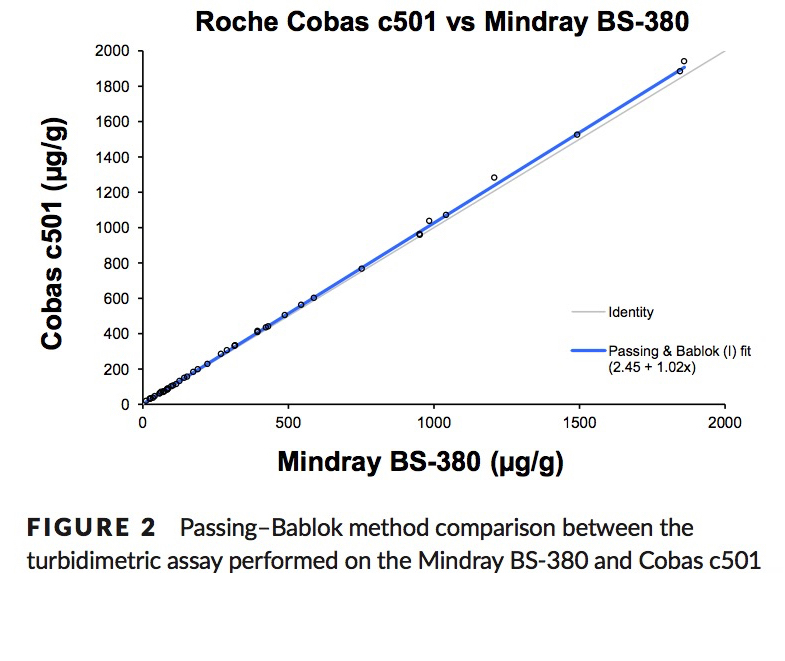
Nilsen, T. et. al. A novel turbidimetric immunoassay for fecal calprotectin optimized for routine chemistry analyzers. J Clin Lab Anal. 2016 Sep 15. PMID:27629827
Method Comparison: fCAL® ELISA vs fCAL® turbo
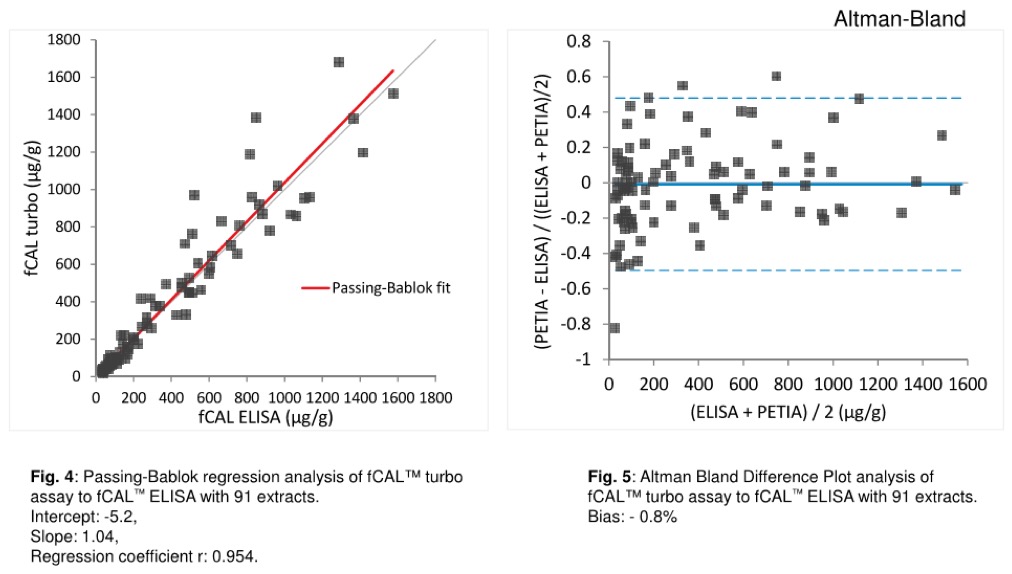
K. Sunde1, M. Schneider2, T. Nilsen1, Ch. Niederberger2, Th. Jermann2, E. Sunderhagen
1Gentian AS, Moss (Norway)
2BÜHLMANN Labortories AG, Schönenbuch (Switzerland)
Learn more about BÜHLMANN fCAL® turbo
BÜHLMANN fCAL® ELISA
BÜHLMANN fCAL® ELISA is the Gold Standard in calprotectin testing
The kit format is a microtiter plate based assay that can be performed manually or on an automated ELISA system to reduce hands-on time.
Two protocols are available with the fecal calprotectin ELISA to cover different ranges.
The ranges are 10-600µg/g or 30-1800µg/g.
All reagents, standards and controls are provided in one kit and are ready for use.
There is no requirement for reconstituting any component, apart from the wash buffer.
This minimizes any errors as well as ensuring a long shelf life.
- Easy-to-use ELISA kit (total assay time is 75 minutes)
- All reagents are ready to use*
- Kit includes calibrators and controls
- Analytical Sensitivity: <10µg/g calprotectin in fecal sample
- Measurable range 30-1800µg/g or 10-600µg/g**
* except wash buffer
** depending on which protocol is used
Learn more about BÜHLMANN fCAL® ELISA
Quantum Blue®
Rapid and Quantitative
- BÜHLMANN’s Quantum Blue® Product line is designed with an innovative solution based on established lateral flow technology which utilizes highly specific monoclonal antibodies to capture the molecule being measured. Optical densities of test and control lines are detected by the Quantum Blue® Reader (small, dedicated reading device) and translated into a quantitative result.
- The Quantum Blue® Reader analyzes line intensities by state of the art image analysis and translates them into a quantitative result within minutes. It is standardized with the BÜHLMANN fCAL® ELISA.
Why Use Quantum Blue®?
- Provides a quantitative measurement of calprotectin in fecal samples
- Simple and rapid extraction and test process
- Results within 12 to 15 minutes
- Excellent correlation with the BÜHLMANN fCAL® ELISA
- Based on established lateral flow technology
- Two kit formats available dependent on test range required:
How to Use Quantum Blue®?
- Convenient and safe stool extraction using CALEX® Cap followed by quantitative fecal calprotectin measurement with the Quantum Blue® fCAL rapid test technology
Quantum Blue® fCAL
You are currently viewing a placeholder content from Youtube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
Comparison of Quantum Blue® to BÜHLMANN fCAL® ELISA
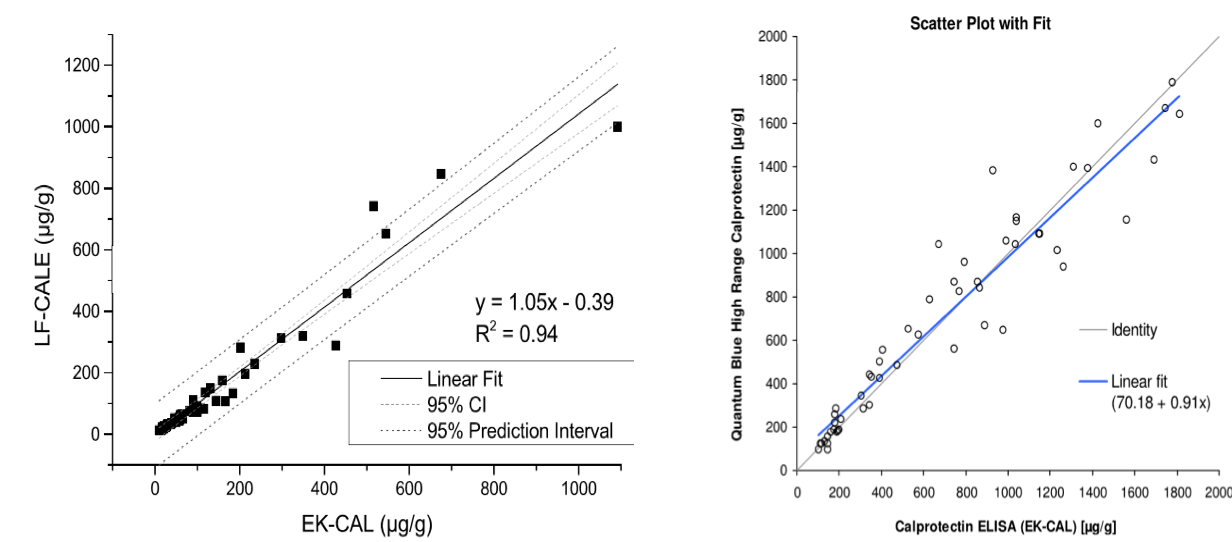
Assaying fCAL on Quantum Blue® LF test cassette/ cartridges (two options):
| Test | Quantum Blue® fCAL Extended | Quantum Blue® fCAL High Range |
|---|---|---|
| Order Code | LF-CALE25 | LF-CHR25 |
| Time to result | 12 min (approx.) | 15 min (approx.) |
| Sample type |
Feces | Feces |
| Standard Range | 30-1000 µg/g | 100-1800 µg/g |
| Sensitivity |
<30 µg/g | <100 µg/g |
| Lot-specific Control Set | B-CALE-CONSET Now included in the kit |
B-CHR-CONSET |
Learn more about Quantum Blue®
IBDoc®
Three Easy Steps to Help Monitor IBD
- Process the sample with the CALEX® Valve extraction device.
- Apply the extracted sample to the calprotectin cassette.
- Use the smartphone CalApp® to read and calculate the result and send it to the gastroenterologist via the secure IBDoc® Portal.
- IBDoc® provides a practical way for more frequent monitoring of patients in the privacy of their own home.
- Under the guidance of clinicians, more regular calprotectin monitoring can help to assess if patients are in prolonged IBD remission and serve as red-flag for increased calprotectin concentrations. This approach allows for a customized, patient-centric approach for IBD disease management.
- IBDoc® is the first self testing application for fecal calprotectin in IBD subjects. Based on BÜHLMANN’s long lasting experience with calprotectin assays, IBDoc® allows IBD subjects for convenient self testing of fecal calprotectin. CALEX® Valve has been developed for simple and easy-to-handle stool extraction. The incorporated valve allows for the application of a precise volume onto the test cassette. The CalApp® smart phone application is available for iOS and Android and transforms smartphones into a test cassette reader.
- For further information, visit www.ibdoc.net or view our IBDoc® videos.
Learn more about IBDoc®




